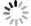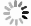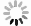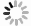Variation patterns of boron and lithium isotopes in salt lakes on the Qinghai–Tibetan Plateau and their application in evaluating resources in the Damxung Co salt lake
-
摘要: 近年,硼、锂同位素地球化学理论和分馏机理的深入,为盐湖体系硼、锂同位素示踪奠定了基础。基于现有大量研究数据,文章系统归纳盐湖体系硼、锂同位素分馏变化特征,总结盐湖演化过程硼、锂同位素组成的变化规律,建立它们的示踪方法。并以此为基础,对西藏典型富硼、锂盐湖−当雄错开展了硼同位素示踪,解决了当雄错与其物源硼同位素特征不符的难题,提出当雄错湖底蕴含大型硼、锂矿床的新认识,并预测了湖底的硼、锂资源量。根据盐湖体系硼、锂同位素地球化学特征,揭示了溶蚀湖的盐湖资源评价意义,为盐湖体系硼、锂同位素示踪和盐湖资源评价奠定理论基础。此外,借助硼同位素地球化学手段建立的当雄错“围岩−地热水−盐湖”的物源补给模式在西藏和全球具有普遍性。Abstract:
Objective The Qinghai–Tibet Plateau is rich in salt lake resources, known particularly for the concentration of elements such as boron and lithium, forming many distinctive resource-type salt lakes. Compared with ordinary salt lakes, a notable characteristic of resource-type salt lakes is the abundant supply of elements such as boron and lithium. Consequently, these elements' sources and accumulation patterns are key scientific issues for understanding the genesis and mineralization patterns of resource-type salt lakes. Boron and lithium isotopes, characterized by significant mass differences and variations in natural isotope ratios, serve as effective tracers for studying the material sources of boron and lithium in salt lakes. However, the application of boron and lithium isotopes in salt lake systems faces the following three challenges: (1) There is insufficient understanding of how boron and lithium isotopes respond to the fundamental geochemical processes of salt lakes. The salt dissolution process that occurs when supply water flows into lake basins is the main reason for drastic changes in geochemical parameters. Inadequate recognition of salt dissolution processes can lead to an overinterpretation of boron and lithium isotope fractionation changes, weakening their tracking capabilities. (2) Isotope fractionation degree is conflated with changes in isotope composition. In salt lake research, discussions of the solid phase's influence on boron and brine's lithium isotopes are often based solely on fractionation factors between the solid and liquid phases, without considering the ratios of boron and lithium amounts involved in the fractionation process. (3) Discrepancies still exist in understanding the fractionation patterns of boron and lithium isotopes during salt crystallization. Methods In light of these problems, our study systematically reviews and analyzes the mechanisms of boron and lithium isotopic fractionation in salt lake systems and summarizes some essential understandings. Conclusion (1) Only salt crystallizations have specific impacts on B and Li isotopes in salt lakes. Since there is a genetic association between salt assemblages and specific salt lake hydrochemical types, the salt lakes with the same hydrochemical type exhibit consistent patterns of B and Li isotope changes during their evolutionary processes. Until halite precipitation, the B and Li isotopic compositions in sulfate- and chloride-type salt lakes are in accord with δ11B and δ7Li values of their sources instead of being controlled by their salt deposits. In contrast, the paths of B and Li isotopic changes of carbonate-type salt lakes are complex and are divided into two branches: calcite subtype and hydromagnesite subtype. After calcite precipitation, the δ11B value of the salt lake increases, and its δ7Li value is marginally above source characteristics (less than 2‰). After hydromagnesite precipitation, the δ11B value of the salt lake is also marginally above source characteristics (less than 2‰). After the stage of halite precipitation, the B and Li isotopic compositions of salt lakes in all types show an increasing trend. (2) Based on the evolutionary processes of B, Li, and K during seawater evaporation, the amounts of B, Li, and K in the current salt lake represent most of the corresponding resources in the lake if the salt lake never experienced complete dryness such as playa. For the salt-dissolving lake, most of the B, Li, and K resources are preserved in salt deposits and interstitial brine at the bottom of the lake. It is optimal for the resource potential of a carbonate-type salt lake in the salt-dissolving lake. (3) The B sources of the current Damxung Co salt lake located in the Tibetan Plateau are from clay carbonates exposed to the lake shore and highly soluble salts and interstitial brine at the bottom of the lake. The geothermal waters produced during early hydrothermal activity are the original B source of the Damxung Co salt lake. Based on mass balance equations, it is estimated that the B resource at the bottom of the Damxung Co salt lake is at least 9.1×106t (B2O3), and the lithium resource is at least 8.6 ×106t (LiCl). -
Key words:
- Qinghai–Tibet Plateau /
- salt lake /
- boron /
- lithium /
- isotopic tracing /
- dissolving lake /
- resource evaluation
-
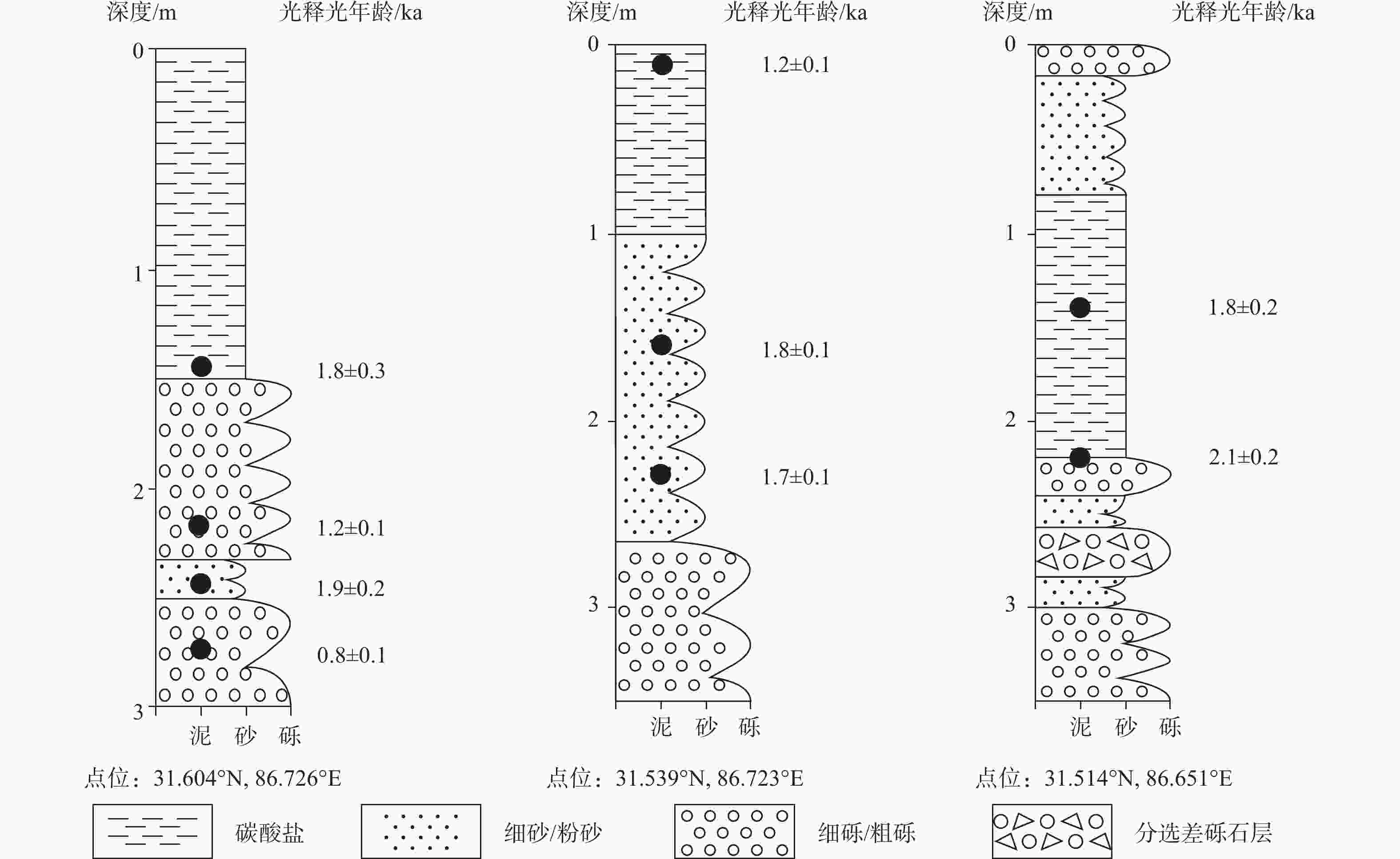
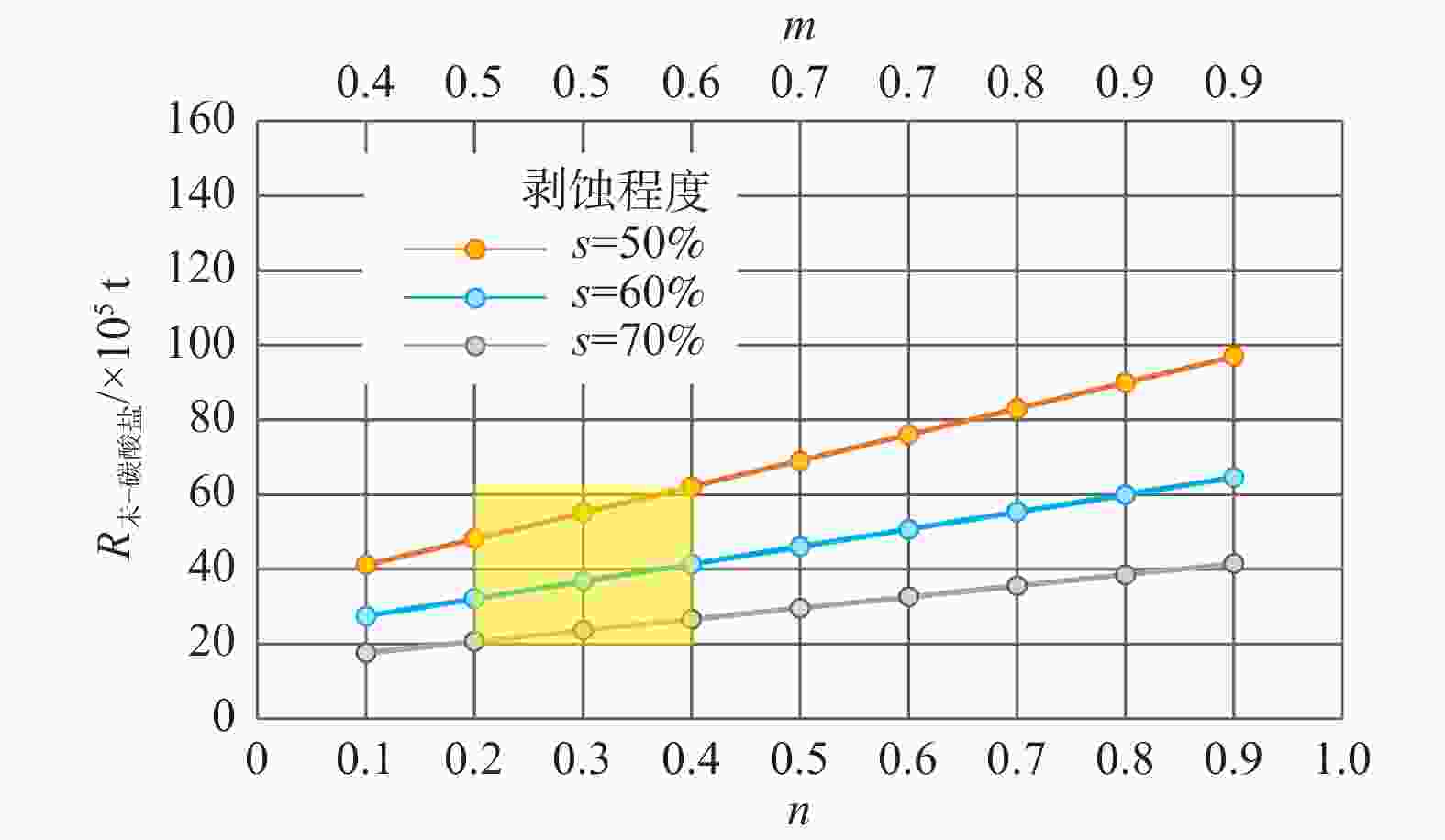
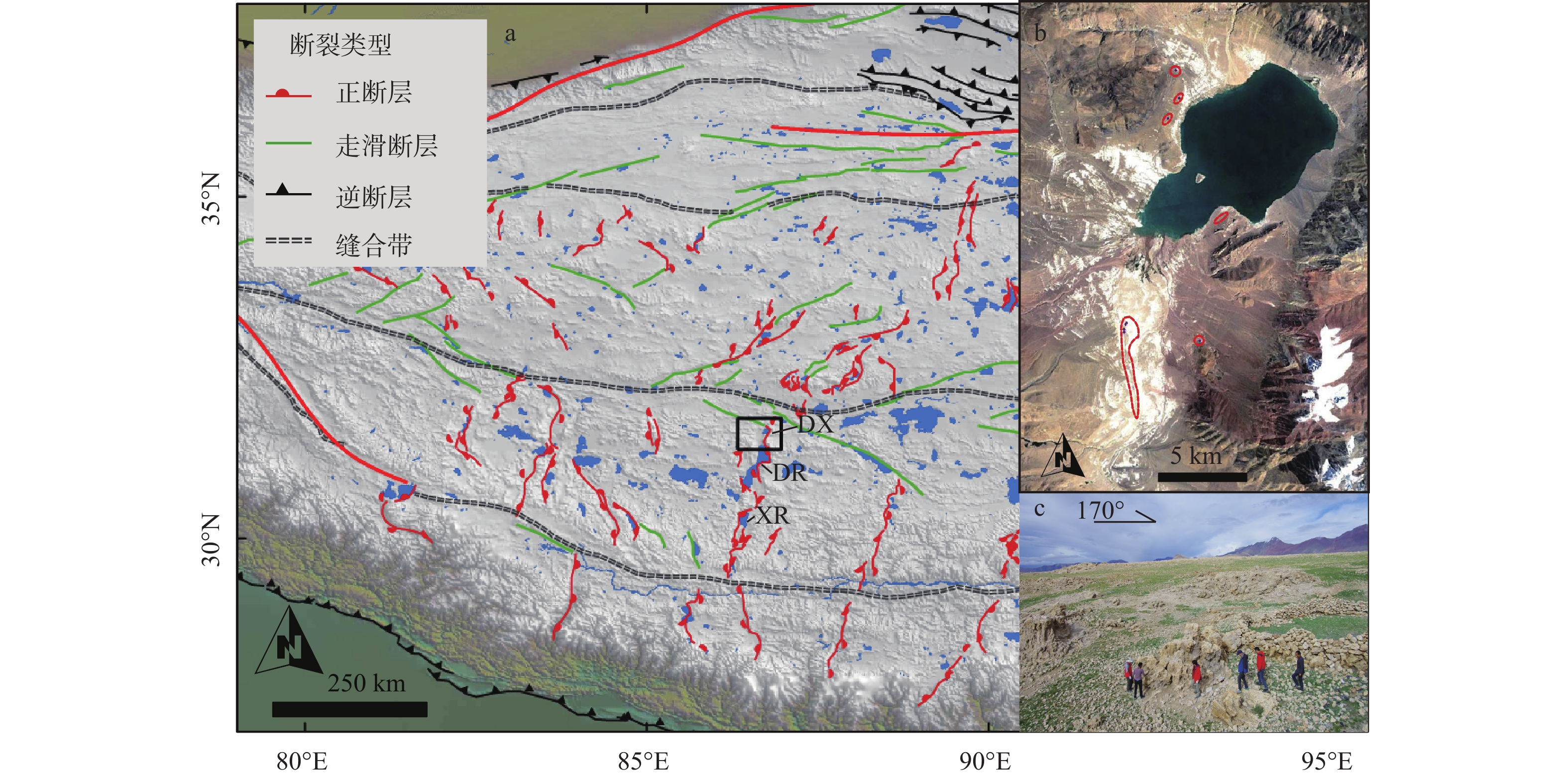
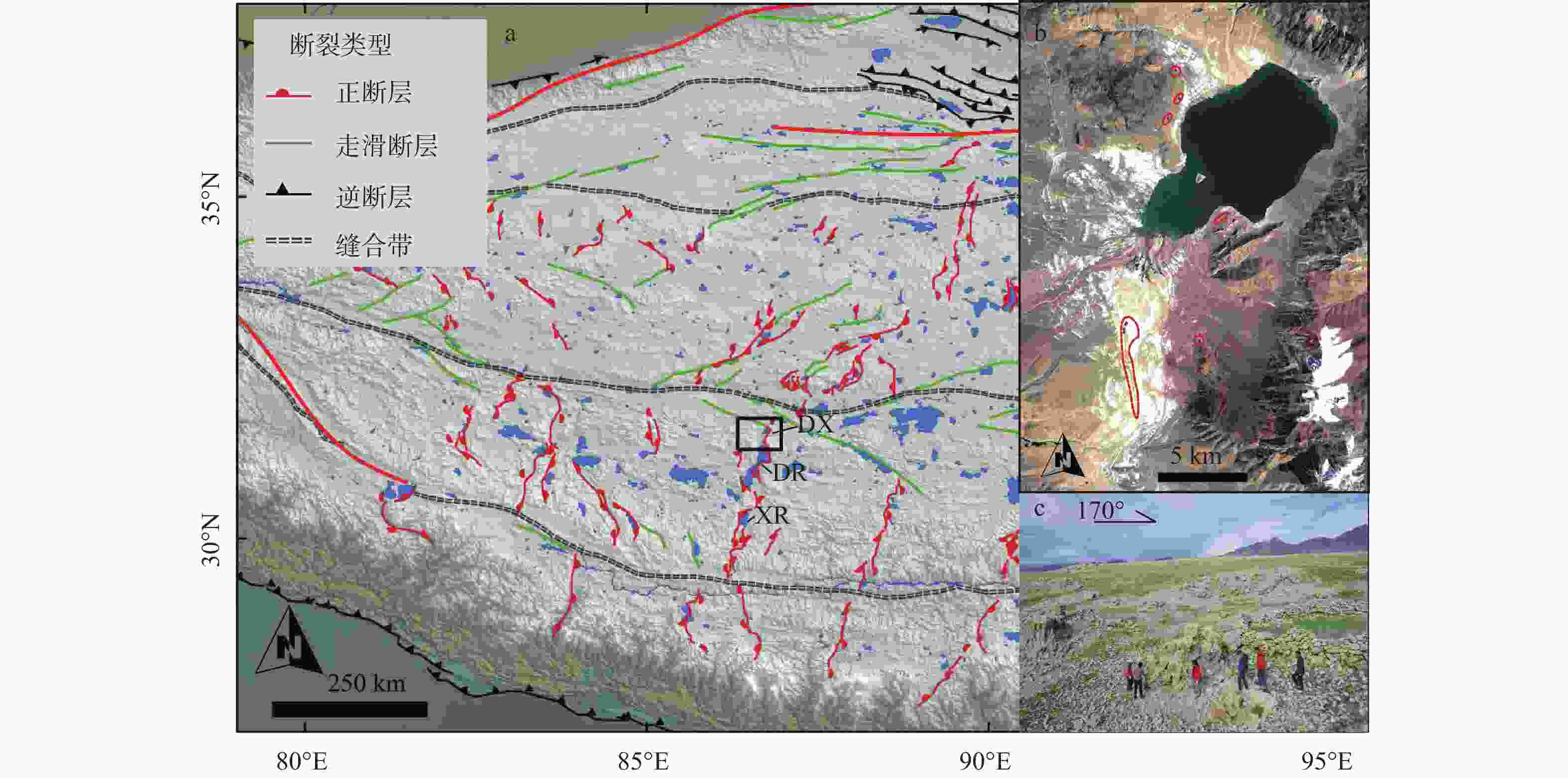
图 1 当雄错构造地质背景与泉华群概况
DX—当雄错盐湖;DR—当若雍措;XR—许如错a—当雄错构造地质背景;b—当雄错泉华分布概况(红线区域指示新、老泉华的分布,影像呈现白色的区域为出露地表的碳酸盐);c—当雄错泉华群野外概况(拍摄位置位于南泉华群一个泉华体顶部)
Figure 1. Tectonic setting of Damxung Co salt lake and overview of the travertine group
(a) Tectonic setting of the Damxung Co salt lake; (b) Overview of travertine distribution in the Damxung Co salt lake (The red lines indicate the distribution of new and old travertines, and the white areas in the image represent exposed carbonate on the surface); (c) Field photo of the travertine outcrops near the Damxung Co lake (taken at one of the travertine top of the southern outcrops) DX−Damxung Co salt lake; DR−Tangra Yumco lake; XR−Xuru Co lake
图 2 当雄错硼同位素地球化学行为与硼矿形成过程.
a—湖水蒸发浓缩至碳酸盐析出阶段;b—干盐滩阶段;c—溶蚀湖阶段
Figure 2. Geochemical behavior of boron isotope and the formation process of boron minerals in the Damxung Co salt lake
(a) Evaporative concentration and carbonate precipitation of the Damxung Co salt lake; (b) Total desiccation of the Damxung Co salt lake; (c) Formation of a salt dissolving lake in the Damxung basin
图 4 随着总碳酸盐沉积的硼矿占比(n)和现今湖水来自碳酸盐的硼占比(m)变化,当雄错未剥蚀碳酸盐的硼矿资源量情况
Figure 4. Boron resource of residual carbonate on lakeshore as a function of proportion of boron in total boron resource derived from carbonate (n), proportion of boron from carbonate erosion in lake water (m) and the degree of carbonate erosion (s)
图 6 随着总碳酸盐沉积的硼矿占比(n)和现今湖水来自碳酸盐的硼占比(m)变化,当雄错湖底未溶蚀的盐沉积和晶间卤水的硼矿资源量情况
Figure 6. Boron resource buried in the bottom of the Damxung Co salt lake as a function of proportion of boron in total boron resource derived from carbonate (n), proportion of boron from carbonate erosion in lake water (m) and the degree of carbonate erosion (s)
表 1 海水和盐湖卤水中黏土矿物吸附的硼同位素分馏系数
Table 1. Boron isotopic fractionation factor (α) during adsorption of boron on clay in seawater and salt lake brine
编号 研究对象 分馏系数α 文献出处 1 海水与沉积物 0.975,0.976 Spivack et al.,1987;Palmer et al.,1987 2 柴达木盐湖−咸水湖与对应沉积物 0.987~0.998 Shirodkar and Xiao,1997;肖应凯等,1999 3 大柴旦卤水与沉积物 0.987~0.992 Xiao et al.,1992 4 卤水与沉积物模拟吸附实验 0.982~0.999 Xiao and Wang,2001(不包括负分馏) 5 尕海盐湖(青海湖旁边)与湖底淤泥 0.985 孙大鹏等,1993 表 2 石盐蒸发实验硼含量和硼同位素组成(据Liu et al.,2000修改)
Table 2. Boron concentrations and isotopic compositions in rock salt evaporation experiments (revised after Liu et al., 2000)
卤水 石盐 α B/ (μg/g) δ11B/ ‰ B/ (μg/g) δ11B/ ‰ 实验-1(纯石盐) 677 10.9 4.4 10.8 0.9999 710 11.2 8.0 10.7 0.9995 1120 11.0 12.2 10.8 0.9999 2871 10.8 39.7 10.9 1.0000 实验-2(含石膏石盐) 585 11.1 6.9 10.8 0.99996 712 10.6 12.4 9.2 0.9987 1038 10.5 17.7 7.1 0.9966 2303 11.0 48.0 7.8 0.9965 表 3 不同水化学类型盐湖的硼、锂同位素变化特征
Table 3. The characteristics of boron and lithium isotopes in salt lakes of different hydrochemical types
水化学类型 主要盐沉积 硼、锂同位素特征 代表盐湖 硫酸盐型 芒硝+石盐 δl=δi 大柴旦,一里坪,东台,西台等 氯化物型 芒硝+石盐 δl=δi 察尔汗,钾湖,巴仑马海等 碳酸盐型 碳酸钙+芒硝+石盐 δ11Bl>δ11Bi;δ7Lil-δ7Lii<2‰ 扎布耶,当雄错 碳酸盐型 碳酸镁+芒硝+石盐 δ11Bl-δ11Bi<2‰;δ7Lil与δ7Lii关系待定 班戈错 表 4 当雄错各类样品硼含量和硼同位素组成
Table 4. Boron concentrations and isotopic compositions of samples from the Damxung Co salt lake
样品类型 B/×10−6 δ11B /‰ 数据来源 地热水 0.40~6.20 −9.8~−8.5 Lü et al.,2013 冷泉水 0.12~0.61 −14.5~-14.1 Lü et al.,2013 河水 0.02~0.15 −4.9~−1.2 Lü et al.,2013 盐湖卤水(0.1~14.6 m) 208.10~1760.80 −18.5~−17.4 北京绵平盐湖研究院,2006;Lü et al.,2013 钙华 90.40~236.00 −29.5~−24.9 Lü et al.,2013 碳酸盐 47.50~264.00 −37.2~−35.3 Lü et al.,2013 表 5 当雄错泉华铀系年龄
Table 5. U-Th results of travertine samples near the Damxung Co salt lake
样品编号 样品类型 238U/×10−9 232Th/×10−9 230Th/232Th/×10−6 δ234U 230Th /238U 230Th年龄/a(未校正) 231Th年龄/a(校正) DXC003-1 泉华 670±2 10076±203 43.4±0.9 452.9±3.2 0.0395±0.0003 3003±23 2702±214 DXC003-15 泉华 302±1 14692±295 13.1±0.3 500.3±3.0 0.0386±0.0006 2836±44 1890±671 DXC003-8 泉华 454±1 8601±173 97.6±2.0 421.4±2.4 0.1122±0.0004 8935±36 8549±275 DXC009-2 泉华 125064±383 59295±1198 1227.8±25.1 521.4±2.5 0.0353±0.0002 2557±12 2548±14 DXC009-14 泉华 46742±165 496200±10093 72.2±1.5 592.3±3.9 0.0465±0.0003 3225±22 3032±139 -
[1] ALLEN K A, HöNISCH B, EGGINS S M, et al. , 2011. Controls on boron incorporation in cultured tests of the planktic foraminifer Orbulina universa[J]. Earth and Planetary Science Letters, 309(3-4): 291-301. doi: 10.1016/j.jpgl.2011.07.010 [2] ANDERSON M A, BERTSCH P M, MILLER W P, 1989. Exchange and apparent fixation of lithium in selected soils and clay minerals[J]. Soil Science, 148(1): 46-52. doi: 10.1097/00010694-198907000-00005 [3] ARAOKA D, KAWAHATA H, TAKAGI T, et al. , 2014. Lithium and strontium isotopic systematics in playas in Nevada, USA: constraints on the origin of lithium[J]. Mineralium Deposita, 49(3): 371-379. doi: 10.1007/s00126-013-0495-y [4] BALAN E, NOIREAUX J, MAVROMATIS V, et al. , 2018. Theoretical isotopic fractionation between structural boron in carbonates and aqueous boric acid and borate ion[J]. Geochimica et Cosmochimica Acta, 222: 117-129. doi: 10.1016/j.gca.2017.10.017 [5] BASSETT R L, 1976. The geochemistry of boron in geothermal waters[D]. Stanford: Stanford University: 128-154. [6] BERGER G, SCHOTT J, GUY C, 1988. Behavior of Li, Rb and Cs during basalt glass and olivine dissolution and chlorite, smectite and zeolite precipitation from seawater: experimental investigations and modelization between 50° and 300℃[J]. Chemical Geology, 71(4): 297-312. doi: 10.1016/0009-2541(88)90056-3 [7] BIAN S, YU Z Q, GONG J F, et al. , 2021. Spatiotemporal distribution and geodynamic mechanism of the nearly NS-trending rifts in the Tibetan Plateau[J]. Journal of Geomechanics, 27(2): 178-194 (in Chinese with English abstract). [8] BRANSON O, 2018. Boron incorporation into marine CaCO3[M]//MARSCHALL H, FOSTER G. Boron isotopes: the fifth element. Cham: Springer: 71-105. [9] CALVET R, PROST R, 1971. Cation migration into empty octahedral sites and surface properties of clays[J]. Clays and Clay Minerals, 19(3): 175-186. doi: 10.1346/CCMN.1971.0190306 [10] CHAN L H, EDMOND J M, 1988. Variation of lithium isotope composition in the marine environment: a preliminary report[J]. Geochimica et Cosmochimica Acta, 52(6): 1711-1717. doi: 10.1016/0016-7037(88)90239-6 [11] CHAN L H, EDMOND J M, THOMPSON G, et al. , 1992. Lithium isotopic composition of submarine basalts: implications for the lithium cycle in the oceans[J]. Earth and Planetary Science Letters, 108(1-3): 151-160. doi: 10.1016/0012-821X(92)90067-6 [12] CHAN L H, LEEMAN W P, PLANK T, 2006. Lithium isotopic composition of marine sediments[J]. Geochemistry, Geophysics, Geosystems, 7(6): Q06005. [13] CHEN K Z, YANG S X, ZHENG X Y, 1981. The salt lakes on the Qinghai-Xizang Plateau[J]. Acta Geographica Sinica, 36(1): 13-21 (in Chinese with English abstract). [14] CHETELAT B, GAILLARDET J, FREYDIER R, et al. , 2005. Boron isotopes in precipitation: Experimental constraints and field evidence from French Guiana[J]. Earth and Planetary Science Letters, 235(1-2): 16-30. doi: 10.1016/j.jpgl.2005.02.014 [15] COCCO G, FANFANI L, ZANAZZI P F, 1978. Lithium[M]//WEDEPOHL K H. Handbook of geochemistry. Berlin: Springer: 3-A-1-3-A-7. [16] DAY C C, POGGE VON STRANDMANN P A E, MASON A J, 2021. Lithium isotopes and partition coefficients in inorganic carbonates: Proxy calibration for weathering reconstruction[J]. Geochimica et Cosmochimica Acta, 305: 243-262. doi: 10.1016/j.gca.2021.02.037 [17] DU Y S, FAN Q S, GAO D L, et al. , 2019. Evaluation of boron isotopes in halite as an indicator of the salinity of Qarhan paleolake water in the eastern Qaidam Basin, western China[J]. Geoscience Frontiers, 10(1): 253-262. doi: 10.1016/j.gsf.2018.02.016 [18] FAN Q S, MA Y Q, CHENG H D, et al. , 2015. Boron occurrence in halite and boron isotope geochemistry of halite in the Qarhan Salt Lake, western China[J]. Sedimentary Geology, 322: 34-42. doi: 10.1016/j.sedgeo.2015.03.012 [19] FARMER J R, BRANSON O, UCHIKAWA J, et al. , 2019. Boric acid and borate incorporation in inorganic calcite inferred from B/Ca, boron isotopes and surface kinetic modeling[J]. Geochimica et Cosmochimica Acta, 244: 229-247. doi: 10.1016/j.gca.2018.10.008 [20] FAURE G, 1991. Principles and applications of inorganic geochemistry: A comprehensive textbook for geology students[M]. New York: Macmillan Publication Co. : 1-251. [21] FÜGER A, KUESSNER M, ROLLION-BARD C, et al. , 2022. Effect of growth rate and pH on Li isotope fractionation during its incorporation in calcite[J]. Geochimica et Cosmochimica Acta, 323: 276-290. doi: 10.1016/j.gca.2022.02.014 [22] GABITOV R I, SCHMITT A K, ROSNER M, et al. , 2011. In situ δ7Li, Li/Ca, and Mg/Ca analyses of synthetic aragonites[J]. Geochemistry, Geophysics, Geosystems, 12(3): A03001,doi: 10.1029/2010GC003322. [23] GAILLARDET J, LEMARCHAND D, GöPEL C, et al. , 2001. Evaporation and sublimation of boric acid: application for boron purification from organic rich solutions[J]. Geostandards and Geoanalytical Research, 25(1): 67-75. doi: 10.1111/j.1751-908X.2001.tb00788.x [24] GAO C L, YU J Q, ZHAN D P, et al. , 2009. Formation and distribution characteristics of boron resource in salt lakes of Qaidam Basin[J]. Journal of Salt Lake Research, 17(4): 6-13 (in Chinese with English abstract). [25] GARCIA M G, BORDA L G, GODFREY L V, et al. , 2020. Characterization of lithium cycling in the Salar De Olaroz, Central Andes, using a geochemical and isotopic approach[J]. Chemical Geology, 531: 119340. doi: 10.1016/j.chemgeo.2019.119340 [26] GAST J A, THOMPSON T G, 1959. Evaporation of boric acid from sea water[J]. Tellus, 11(3): 344-347. doi: 10.3402/tellusa.v11i3.9313 [27] GODFREY L V, CHAN L H, ALONSO R N, et al. , 2013. The role of climate in the accumulation of lithium-rich brine in the Central Andes[J]. Applied Geochemistry, 38: 92-102. doi: 10.1016/j.apgeochem.2013.09.002 [28] GOLDBERG S, GLAUBIG R A, 1986. Boron adsorption and silicon release by the clay minerals kaolinite, Montmorillonite, and illite[J]. Soil Science Society of America Journal, 50(6): 1442-1448. doi: 10.2136/sssaj1986.03615995005000060013x [29] GOLDBERG S, FORSTER H S, HEICK E L, 1993a. Boron adsorption mechanisms on oxides, clay minerals, and soils inferred from ionic strength effects[J]. Soil Science Society of America Journal, 57(3): 704-708. doi: 10.2136/sssaj1993.03615995005700030013x [30] GOLDBERG S, FORSTER H S, HEICK E L, 1993b. Temperature effects on boron adsorption by reference minerals and soils[J]. Soil Science, 156(5): 316-321. doi: 10.1097/00010694-199311000-00004 [31] GOTO A, ARAKAWA H, MORINAGA H, et al. , 2003. The occurrence of hydromagnesite in bottom sediments from Lake Siling, central Tibet: implications for the correlation among δ18O, δ13C and particle density[J]. Journal of Asian Earth Sciences, 21(9): 979-988. doi: 10.1016/S1367-9120(02)00169-4 [32] GU H E, MA Y Q, PENG Z K, et al. , 2023. Influence of polyborate ions on the fractionation of B isotopes during calcite deposition[J]. Chemical Geology, 622: 121387. doi: 10.1016/j.chemgeo.2023.121387 [33] HE M Y, XIAO Y K, JIN Z D, et al. , 2013. Quantification of boron incorporation into synthetic calcite under controlled pH and temperature conditions using a differential solubility technique[J]. Chemical Geology, 337-338: 67-74. doi: 10.1016/j.chemgeo.2012.11.013 [34] HE M Y, LUO C G, YANG H J, et al. , 2020. Sources and a proposal for comprehensive exploitation of lithium brine deposits in the Qaidam Basin on the northern Tibetan Plateau, China: Evidence from Li isotopes[J]. Ore Geology Reviews, 117: 103277. doi: 10.1016/j.oregeorev.2019.103277 [35] HEMMING N G, HANSON G N, 1992. Boron isotopic composition and concentration in modern marine carbonates[J]. Geochimica et Cosmochimica Acta, 56(1): 537-543. doi: 10.1016/0016-7037(92)90151-8 [36] HEMMING N G, HANSON G N, 1994. A procedure for the isotopic analysis of boron by negative thermal ionization mass spectrometry[J]. Chemical Geology, 114(1-2): 147-156. doi: 10.1016/0009-2541(94)90048-5 [37] HEMMING N G, REEDER R J, HANSON G N, 1995. Mineral-fluid partitioning and isotopic fractionation of boron in synthetic calcium carbonate[J]. Geochimica et Cosmochimica Acta, 59(2): 371-379. doi: 10.1016/0016-7037(95)00288-B [38] HEMMING N G, REEDER R J, HART S R, 1998. Growth-step-selective incorporation of boron on the calcite surface[J]. Geochimica et Cosmochimica Acta, 62(17): 2915-2922. doi: 10.1016/S0016-7037(98)00214-2 [39] HENEHAN M J, GEBBINCK C D K, WYMAN J V B, et al. , 2022. No ion is an island: multiple ions influence boron incorporation into CaCO3[J]. Geochimica et Cosmochimica Acta, 318: 510-530. doi: 10.1016/j.gca.2021.12.011 [40] HINDSHAW R S, TOSCA R, GOûT T L, et al. , 2019. Experimental constraints on Li isotope fractionation during clay formation[J]. Geochimica et Cosmochimica Acta, 250: 219-237. doi: 10.1016/j.gca.2019.02.015 [41] HINGSTON F J, POSNER A M, QUIRK J P, 1972. Anion adsorption by goethite and gibbsite[J]. Journal of Soil Science, 23(2): 177-192. doi: 10.1111/j.1365-2389.1972.tb01652.x [42] HUH Y, CHAN L H, ZHANG L B, et al. , 1998. Lithium and its isotopes in major world rivers: Implications for weathering and the oceanic budget[J]. Geochimica et Cosmochimica Acta, 62(12): 2039-2051. doi: 10.1016/S0016-7037(98)00126-4 [43] KACZMAREK K, NEHRKE G, MISRA S, et al. , 2016. Investigating the effects of growth rate and temperature on the B/Ca ratio and δ11B during inorganic calcite formation[J]. Chemical Geology, 421: 81-92. doi: 10.1016/j.chemgeo.2015.12.002 [44] KASEMANN S A, MEIXNER A, ERZINGER J, et al. , 2004. Boron isotope composition of geothermal fluids and borate minerals from salar deposits (central Andes/NW Argentina)[J]. Journal of South American Earth Sciences, 16(8): 685-697. doi: 10.1016/j.jsames.2003.12.004 [45] KEREN R, MEZUMAN U, 1981. Boron adsorption by clay minerals using a phenomenological equation[J]. Clays and Clay Minerals, 29(3): 198-204. doi: 10.1346/CCMN.1981.0290305 [46] KOBAYASHI K, HASHIMOTO Y, WANG S L, 2020. Boron incorporation into precipitated calcium carbonates affected by aqueous pH and boron concentration[J]. Journal of Hazardous Materials, 383: 121183. doi: 10.1016/j.jhazmat.2019.121183 [47] LÉCUYER C, GRANDJEAN P, REYNARD B, et al. , 2002. 11B/10B analysis of geological materials by ICP–MS Plasma 54: Application to the boron fractionation between brachiopod calcite and seawater[J]. Chemical Geology, 186(1-2): 45-55. doi: 10.1016/S0009-2541(01)00425-9 [48] LI B K, CHENG H D, MA H Z, 2022a. Boron isotope geochemistry of the lakkor co salt lake (Tibet) and its geological significance[J]. Geofluids, 2022: 3724800. [49] LI B K, HE M Y, MA H Z, et al. , 2022b. Boron isotope geochemistry of Bangor Co Salt Lake (central Tibet): implications for boron origin and uneven mixing of lake water[J]. Acta Geochimica, 41(5): 731-740. doi: 10.1007/s11631-022-00542-1 [50] LI J S, CHEN F K, LING Z Y, et al. , 2021. Lithium sources in oilfield waters from the Qaidam Basin, Tibetan Plateau: Geochemical and Li isotopic evidence[J]. Ore Geology Reviews, 139: 104481. doi: 10.1016/j.oregeorev.2021.104481 [51] LI J Y, 1994. Distributive regularity of boron and lithium in Da Qaidam Salt Lake[J]. Journal of Salt Lake Research, 2(2): 20-28 (in Chinese with English abstract). [52] LI W S, LIU X M, 2020. Experimental investigation of lithium isotope fractionation during kaolinite adsorption: Implications for chemical weathering[J]. Geochimica et Cosmochimica Acta, 284: 156-172. doi: 10.1016/j.gca.2020.06.025 [53] LI W S, LIU X M, 2022. Mineralogy and fluid chemistry controls on lithium isotope fractionation during clay adsorption[J]. Science of the Total Environment, 851: 158138. doi: 10.1016/j.scitotenv.2022.158138 [54] LIN Y J, ZHENG M P, YE C Y, 2017. Hydromagnesite precipitation in the Alkaline Lake Dujiali, central Qinghai-Tibetan Plateau: Constraints on hydromagnesite precipitation from hydrochemistry and stable isotopes[J]. Applied Geochemistry, 78: 139-148. doi: 10.1016/j.apgeochem.2016.12.020 [55] LIN Y J, ZHENG M P, YE C Y, et al. , 2019. Trace and rare earth element geochemistry of Holocene hydromagnesite from Dujiali Lake, central Qinghai–Tibetan Plateau, China[J]. Carbonates and Evaporites, 34(4): 1265-1279. doi: 10.1007/s13146-017-0395-9 [56] LIU W G, XIAO Y K, PENG Z C, 1999. Relimiary study of hydrochemistry characteristics of boron and chlorine isotopes of salt lake brines in Qaidam Basin[J]. Journal of Salt Lake Research, 7(3): 8-14 (in Chinese with English abstract). [57] LIU W G, XIAO Y K, PENG Z C, et al. , 2000. Boron concentration and isotopic composition of halite from experiments and salt lakes in the Qaidam Basin[J]. Geochimica et Cosmochimica Acta, 64(13): 2177-2183. doi: 10.1016/S0016-7037(00)00363-X [58] LIU X F, ZHENG M P, QI W, 2007. Sources of ore-forming materials of the superlarge B and Li deposit in Zabuye Salt Lake, Tibet, China[J]. Acta Geologica Sinica, 81(12): 1709-1715 (in Chinese with English abstract). [59] LONG H, LAI Z P, FRENZEL P, et al. , 2012. Holocene moist period recorded by the chronostratigraphy of a lake sedimentary sequence from Lake Tangra Yumco on the south Tibetan Plateau[J]. Quaternary Geochronology, 10: 136-142. doi: 10.1016/j.quageo.2011.11.005 [60] LÓPEZ STEINMETZ R L, 2017. Lithium- and boron-bearing brines in the Central Andes: exploring hydrofacies on the eastern Puna plateau between 23° and 23°30′S[J]. Miner Deposita, 52(1): 35-50. doi: 10.1007/s00126-016-0656-x [61] LÓPEZ STEINMETZ R L, SALVI S, GARCÍA M G, et al. , 2018. Northern Puna Plateau-scale survey of Li brine-type deposits in the Andes of NW Argentina[J]. Journal of Geochemical Exploration, 190: 26–38. LU S C, MA Y Q, LÜ S, et al. , 2022. Systematic boron isotope analysis on a Quaternary deep SG-1 core from the Qaidam Basin, NE Tibetan Plateau and its paleoclimate implication[J]. Quaternary International, 631: 1-10. doi: 10.1016/j.quaint.2022.04.014 [62] LÜ Y Y, 2008. Determination of Boron isotopes by MC-ICPMS and its application to the Tibetan geotherms and salt lakes[D]. Beijing: Institute of Geology and Geophysics, Chinese Academy of Sciences: 1-113 (in Chinese). [63] LÜ Y Y, ZHENG M P, CHEN W X, et al. , 2013. Origin of boron in the Damxung Co Salt Lake (central Tibet): evidence from boron geochemistry and isotopes[J]. Geochemical Journal, 47(5): 513-523. doi: 10.2343/geochemj.2.0273 [64] LU S C, MA Y Q, LÜ S, et al., 2022. Systematic boron isotope analysis on a Quaternary deep SG-1 core from the Qaidam Basin, NE Tibetan Plateau and its paleoclimate implication[J]. Quaternary International, 631: 1-10. [65] MA R Y, HAN F Q, MA H Z, et al. , 2015. Hydrochemical characteristics and boron isotope geochemistry of brine in Hoh Xil, Qinghai Province[J]. Acta Geoscientica Sinica, 36(1): 60-66 (in Chinese with English abstract). [66] MARRIOTT C S, HENDERSON G M, BELSHAW N S, et al. , 2004a. Temperature dependence of δ7Li, δ44Ca and Li/Ca during growth of calcium carbonate[J]. Earth and Planetary Science Letters, 222(2): 615-624. doi: 10.1016/j.jpgl.2004.02.031 [67] MARRIOTT C S, HENDERSON G M, CROMPTON R, et al. , 2004b. Effect of mineralogy, salinity, and temperature on Li/Ca and Li isotope composition of calcium carbonate[J]. Chemical Geology, 212(1-2): 5-15. doi: 10.1016/j.chemgeo.2004.08.002 [68] MAVROMATIS V, MONTOUILLOUT V, NOIREAUX J, et al. , 2015. Characterization of boron incorporation and speciation in calcite and aragonite from co-precipitation experiments under controlled pH, temperature and precipitation rate[J]. Geochimica et Cosmochimica Acta, 150: 299-313. doi: 10.1016/j.gca.2014.10.024 [69] MAVROMATIS V, PURGSTALLER B, LOUVAT P, et al. , 2021. Boron isotope fractionation during the formation of amorphous calcium carbonates and their transformation to Mg-calcite and aragonite[J]. Geochimica et Cosmochimica Acta, 315: 152-171. doi: 10.1016/j.gca.2021.08.041 [70] MIAO W L, ZHANG X Y, LI Y L, et al. , 2022. Lithium and strontium isotopic systematics in the Nalenggele River catchment of Qaidam basin, China: Quantifying contributions to lithium brines and deciphering lithium behavior in hydrological processes[J]. Journal of Hydrology, 614: 128630. doi: 10.1016/j.jhydrol.2022.128630 [71] MILLOT R, GIRARD J P, 2007. Lithium isotope fractionation during adsorption onto mineral surfaces[C]//Clay in natural & engineered barriers for radioactive waste confinement - 3rd international meeting. Lille, 307-308. [72] MILLOT R, PETELET-GIRAUD E, GUERROT C, et al. , 2010. Multi-isotopic composition (δ7Li–δ11B–δD–δ18O) of rainwaters in France: Origin and spatio-temporal characterization[J]. Applied Geochemistry, 25(10): 1510-1524. doi: 10.1016/j.apgeochem.2010.08.002 [73] MUNK L A, BOUTT D F, HYNEK S A, et al. , 2018. Hydrogeochemical fluxes and processes contributing to the formation of lithium-enriched brines in a hyper-arid continental basin[J]. Chemical Geology, 493: 37-57. doi: 10.1016/j.chemgeo.2018.05.013 [74] Institute of Mineral Resources, Chinese Academy of Geological Sciences, 2024. Report on enrichment and crystallization processes of potassium salt and brine lithium deposits in China[R]. (in Chinese) [75] NOIREAUX J, MAVROMATIS V, GAILLARDET J, et al. , 2015. Crystallographic control on the boron isotope paleo-pH proxy[J]. Earth and Planetary Science Letters, 430: 398-407. doi: 10.1016/j.jpgl.2015.07.063 [76] OI T, NOMURA M, MUSASHI M, et al. , 1989. Boron isotopic compositions of some boron minerals[J]. Geochimica et Cosmochimica Acta, 53(12): 3189-3195. doi: 10.1016/0016-7037(89)90099-9 [77] PAGANI M, LEMARCHAND D, SPIVACK A, et al. , 2005. A critical evaluation of the boron isotope-pH proxy: The accuracy of ancient ocean pH estimates[J]. Geochimica et Cosmochimica Acta, 69(4): 953-961. doi: 10.1016/j.gca.2004.07.029 [78] PALMER M R, SPIVACK A J, EDMOND J M, 1987. Temperature and pH controls over isotopic fractionation during adsorption of boron on marine clay[J]. Geochimica et Cosmochimica Acta, 51(9): 2319-2323. doi: 10.1016/0016-7037(87)90285-7 [79] PALMER M R, LONDON D, MORGAN G B, et al. , 1992. Experimental determination of fractionation of 11B/10B between tourmaline and aqueous vapor: A temperature- and pressure-dependent isotopic system[J]. Chemical Geology: Isotope Geoscience Section, 101(1-2): 123-129. doi: 10.1016/0009-2541(92)90209-N [80] PISTINER J S, HENDERSON G M, 2003. Lithium-isotope fractionation during continental weathering processes[J]. Earth and Planetary Science Letters, 214(1-2): 327-339. doi: 10.1016/S0012-821X(03)00348-0 [81] POGGE VON STRANDMANN P A E, VAKS A, BAR-MATTHEWS M, et al. , 2017. Lithium isotopes in speleothems: Temperature-controlled variation in silicate weathering during glacial cycles[J]. Earth and Planetary Science Letters, 469: 64-74. doi: 10.1016/j.jpgl.2017.04.014 [82] POGGE VON STRANDMANN P A E, SCHMIDT D N, PLANAVSKY N J, et al. , 2019. Assessing bulk carbonates as archives for seawater Li isotope ratios[J]. Chemical Geology, 530: 119338. doi: 10.1016/j.chemgeo.2019.119338 [83] QI H P, WANG Y H, XIAO Y K, et al. , 1993. A preliminary study of boron isotopes in salt lakes of China[J]. Chinese Science Bulletin, 38(7): 634-637 (in Chinese). doi: 10.1360/csb1993-38-7-634 [84] QING D L, MA H Z, LI B K, 2012. Boron concentration and isotopic fractionation research in BangkogCo intercrystal brine evaporation process[J]. Journal of Salt Lake Research, 20(3): 15-20 (in Chinese with English abstract). [85] RADES E F, TSUKAMOTO S, FRECHEN M, et al. , 2015. A lake-level chronology based on feldspar luminescence dating of beach ridges at Tangra Yum Co (southern Tibet)[J]. Quaternary Research, 83(3): 469-478. doi: 10.1016/j.yqres.2015.03.002 [86] SALDI G D, NOIREAUX J, LOUVAT P, et al. , 2018. Boron isotopic fractionation during adsorption by calcite – Implication for the seawater pH proxy[J]. Geochimica et Cosmochimica Acta, 240: 255-273. doi: 10.1016/j.gca.2018.08.025 [87] SANYAL A, NUGENT M, REEDER R J, et al. , 2000. Seawater pH control on the boron isotopic composition of calcite: evidence from inorganic calcite precipitation experiments[J]. Geochimica et Cosmochimica Acta, 64(9): 1551-1555. doi: 10.1016/S0016-7037(99)00437-8 [88] SEYEDALI M, COOGAN L A, GILLIS K M, 2021. The effect of solution chemistry on elemental and isotopic fractionation of lithium during inorganic precipitation of calcite[J]. Geochimica et Cosmochimica Acta, 311: 102-118. doi: 10.1016/j.gca.2021.07.021 [89] SHIRODKAR P V, XIAO Y K, 1997. Isotopic compositions of boron in sediments and their implications[J]. Current Science, 72(1): 74-77. [90] SONG H B, LI Y W, 1994. Indoor evaporation experiment on water of South China Sea[J]. Acta Geoscientia Sinica, 15(1-2): 157-167 (in Chinese with English abstract). [91] SPIVACK A J, EDMOND J M, 1987. Boron isotope exchange between seawater and the oceanic crust[J]. Geochimica et Cosmochimica Acta, 51(5): 1033-1043. doi: 10.1016/0016-7037(87)90198-0 [92] SPIVACK A J, PALMER M R, EDMOND J M, 1987. The sedimentary cycle of the boron isotopes[J]. Geochimica et Cosmochimica Acta, 51(7): 1939-1949. doi: 10.1016/0016-7037(87)90183-9 [93] STOFFYNEGLI P, MACKENZIE F T, 1984. Mass balance of dissolved lithium in the oceans[J]. Geochimica et Cosmochimica Acta, 48(4): 859-872. doi: 10.1016/0016-7037(84)90107-8 [94] SUN D P, 1991. Origin of borates in Xiao-Chaidan Lake, Chaidam basin, China[J]. Mineralogy and Petrology, 11(4): 57-65 (in Chinese with English abstract). [95] SUN D P, TANG Y, XU Z Q, et al. , 1991. A preliminary study of hydrochemical evolution in Lake Qinghai of Qaidam basin, China[J]. Chinese Science Bulletin, 36(15): 1172-1174 (in Chinese). doi: 10.1360/csb1991-36-15-1172 [96] SUN D P, XIAO Y K, WANG Y H, et al. , 1993. A preliminary study of boron isotopes in Lake Qinghai of Qaidam basin, China[J]. Chinese Science Bulletin, 38(9): 822-825 (in Chinese). doi: 10.1360/csb1993-38-9-822 [97] SWIHART G H, MOORE P B, CALLIS E L, 1986. Boron isotopic composition of marine and nonmarine evaporite borates[J]. Geochimica et Cosmochimica Acta, 50(6): 1297-1301. doi: 10.1016/0016-7037(86)90413-8 [98] TAN H B, CHEN J, RAO W B, et al. , 2012. Geothermal constraints on enrichment of boron and lithium in salt lakes: an example from a river-salt lake system on the northern slope of the eastern Kunlun Mountains, China[J]. Journal of Asian Earth Sciences, 51: 21-29. doi: 10.1016/j.jseaes.2012.03.002 [99] TANG Y J, ZHANG H F, YING J F, 2007. Review of the lithium isotope system as a geochemical tracer[J]. International Geology Review, 49: 374-388. doi: 10.2747/0020-6814.49.4.374 [100] TARDY Y, KREMPP G, TRAUTH N, 1972. Le lithium dans les minéraux argileux des sédiments et des sols[J]. Geochimica et Cosmochimica Acta, 36(4): 397-412. doi: 10.1016/0016-7037(72)90031-2 [101] TOMASCAK P B, HEMMING N G, HEMMING S R, 2003. The lithium isotopic composition of waters of the Mono Basin, California[J]. Geochimica et Cosmochimica Acta, 67(4): 601-611. doi: 10.1016/S0016-7037(02)01132-8 [102] TOMASCAK P B, 2004. Developments in the understanding and application of lithium isotopes in the earth and planetary sciences[J]. Reviews in Mineralogy and Geochemistry, 55(1): 153-195. doi: 10.2138/gsrmg.55.1.153 [103] TOMASCAK P B, MAGNA T, DOHMEN R, 2016. Advances in lithium isotope geochemistry[M]. Cham: Springer: 1-195. [104] TONG W, ZHANG M T, ZHANG Z F, et al. , 1981. Geothermals beneath Xizang (Tibetan) Plateau[M]. Beijing: Science Press: 1-170 (in Chinese). [105] TUCKER M E, WRIGHT V P, 1990. Carbonate depositional systems i: marine shallow-water and lacustrine carbonates[M]//TUCKER M E, WRIGHT V P. Carbonate sedimentology. Oxford: Blackwell Science: 101-227. [106] VENGOSH A, CHIVAS A R, MCCULLOCH M T, et al. , 1991a. Boron isotope geochemistry of Australian salt lakes[J]. Geochimica et Cosmochimica Acta, 55(9): 2591-2606. doi: 10.1016/0016-7037(91)90375-F [107] VENGOSH A, STARINSKY A, KOLODNY Y, et al. , 1991b. Boron isotope geochemistry as a tracer for the evolution of brines and associated hot springs from the Dead Sea, Israel[J]. Geochimica et Cosmochimica Acta, 55(6): 1689-1695. doi: 10.1016/0016-7037(91)90139-V [108] VENGOSH A, STARINSKY A, KOLODNY Y, et al. , 1992. Boron isotope variations during fractional evaporation of sea water: New constraints on the marine vs. nonmarine debate[J]. Geology, 20(9): 799-802. doi: 10.1130/0091-7613(1992)020<0799:BIVDFE>2.3.CO;2 [109] VENGOSH A, CHIVAS A R, STARINSKY A, et al. , 1995. Chemical and boron isotope compositions of non-marine brines from the Qaidam Basin, Qinghai, China[J]. Chemical Geology, 120(1-2): 135-154. doi: 10.1016/0009-2541(94)00118-R [110] VIGIER N, DECARREAU A, MILLOT R, et al. , 2008. Quantifying Li isotope fractionation during smectite formation and implications for the Li cycle[J]. Geochimica et Cosmochimica Acta, 72(3): 780-792. doi: 10.1016/j.gca.2007.11.011 [111] VINE J D, COLO D, 1975. Lithium in sediments and brines-how, why, and where to search[J]. Journal of Research of the U. S. Geological Survey, 3(4): 479-485. [112] WANG Q Z, XIAO Y K, ZHANG C G, et al. , 2001. Boron isotopic compositions of some boron minerals in Qinghai and Tibet[J]. Bulletin of Mineralogy, Petrology and Geochemistry, 20(4): 364-366 (in Chinese with English abstract). [113] WANG S M, DOU H S, 1998. Records of Chinese lakes[M]. Beijing: Science Press: 1-580 (in Chinese). [114] WANG X D, LIU C Q, ZHAO Z Q, et al. , 2017. Boron isotope geochemistry of Zigetang Co saline lake sediments, Tibetan Plateau[J]. Acta Geochimica, 36(3): 437-439. doi: 10.1007/s11631-017-0185-z [115] WANG Y J, WEI H Z, JIANG S Y, et al. , 2018. Mechanism of boron incorporation into calcites and associated isotope fractionation in a steady-state carbonate-seawater system[J]. Applied Geochemistry, 98: 221-236. doi: 10.1016/j.apgeochem.2018.09.013 [116] WEI H Z, JIANG S Y, TAN H B, et al. , 2014. Boron isotope geochemistry of salt sediments from the Dongtai salt lake in Qaidam Basin: Boron budget and sources[J]. Chemical Geology, 380: 74-83. doi: 10.1016/j.chemgeo.2014.04.026 [117] WEYNELL M, WIECHERT U, SCHUESSLER J A, 2017. Lithium isotopes and implications on chemical weathering in the catchment of Lake Donggi Cona, northeastern Tibetan Plateau[J]. Geochimica et Cosmochimica Acta, 213: 155-177. doi: 10.1016/j.gca.2017.06.026 [118] WEYNELL M, WIECHERT U, SCHUESSLER J A, 2021. Lithium isotope signatures of weathering in the hyper-arid climate of the western Tibetan Plateau[J]. Geochimica et Cosmochimica Acta, 293: 205-223. doi: 10.1016/j.gca.2020.10.021 [119] WU L L, MA W Z, TANG Y, 1984. On the water-chemical properties and formative conditions of high-boron brine in Qinghai-Xizang Plateau[J]. Geographical Research, 3(4): 1-11 (in Chinese with English abstract). [120] WU Q, ZHENG M P, NIE Z, et al. , 2012. Natural evaporation and crystallization regularity of Dangxiongcuo carbonate-type salt lake brine in Tibet[J]. Chinese Journal of Inorganic Chemistry, 28(9): 1895-1903 (in Chinese with English abstract). [121] WU Q, ZHENG M P, NIE Z, et al. , 2013. Experiment study of solar evaporation of brine from the Dangxiongcuo Salt Lake in Tibet in winter[J]. Acta Geologica Sinica, 87(3): 433-440 (in Chinese with English abstract). [122] WU Y Q, ZHAO Z Q, 2011. Experimental study on the adsorption of Li+ on kaolinite and montmorillonite[J]. Acta Mineralogica Sinica, 31(2): 291-295 (in Chinese with English abstract). [123] WU Z H, ZHANG Y S, HU D G, et al. , 2007. Late Cenozoic normal faulting of the Qungdo’Gyang Graben in the central segment of the Cona-Oiga Rift, southeastern Tibet[J]. Journal of Geomechanics, 13(4): 297-306 (in Chinese with English abstract). [124] WU Z M, CUI X M, ZHENG M P, 2012. pH value change trends in salt brine evaporation[J]. Chinese Journal of Inorganic Chemistry, 28(2): 297-301 (in Chinese with English abstract). [125] XIAO J, XIAO Y K, LIU C Q, et al. , 2009. Boron isotopic fractionation during incorporation of boron into Mg(OH)2[J]. Chinese Science Bulletin, 54(17): 3090-3100. doi: 10.1007/s11434-009-0138-y [126] XIAO J, XIAO Y K, JIN Z D, et al. , 2013. Boron isotope variations and its geochemical application in nature[J]. Australian Journal of Earth Sciences, 60(4): 431-447. doi: 10.1080/08120099.2013.813585 [127] XIAO Y K, SUN D P, WANG Y H, et al. , 1992. Boron isotopic compositions of brine, sediments, and source water in Da Qaidam Lake, Qinghai, China[J]. Geochimica et Cosmochimica Acta, 56(4): 1561-1568. doi: 10.1016/0016-7037(92)90225-8 [128] XIAO Y K, QI H P, WANG Y H, et al. , 1994. lithium isotopic compositions of brine, sediments and source water in da Qaidam lake, Qinghai, China[J]. Geochimica, 23(4): 329-338 (in Chinese with English abstract). [129] XIAO Y K, SHIRODKAR P V, LIU W G, et al. , 1999. The investigation on isotopic geochemistry of boron in salt lake, Qaidam Basin, Qinghai[J]. Progress in Natural Science, 9(7): 612-618 (in Chinese). [130] XIAO Y K, SWIHART G H, XIAO Y, et al. , 2001. A preliminary experimental study of the boron concentration in vapor and the isotopic fractionation of boron between seawater and vapor during evaporation of seawater[J]. Science in China Series B: Chemistry, 44(5): 540-551. doi: 10.1007/BF02880685 [131] XIAO Y K, WANG L, 2001. The effect of pH and temperature on the isotopic fractionation of boron between saline brine and sediments[J]. Chemical Geology, 171(3-4): 253-261. doi: 10.1016/S0009-2541(00)00251-5 [132] XIAO Y K, LI S Z, WEI H Z, et al. , 2006. An unusual isotopic fractionation of boron in synthetic calcium carbonate precipitated from seawater and saline water[J]. Science in China Series B: Chemistry, 49(5): 454-465. [133] XIAO Y K, LI S Z, WEI H Z, et al. , 2007. Boron isotopic fractionation during seawater evaporation[J]. Marine Chemistry, 103(3-4): 382-392. doi: 10.1016/j.marchem.2006.10.007 [134] XIAO Y K, LI H L, LIU W G, et al. , 2008. Boron isotopic fractionation in laboratory inorganic carbonate precipitation: Evidence for the incorporation of B(OH)3 into carbonate[J]. Science in China Series D: Earth Sciences, 51(12): 1776-1785. doi: 10.1007/s11430-008-0144-y [135] YAMAJI K, MAKITA Y, WATANABE H, et al. , 2001. Theoretical estimation of lithium isotopic reduced partition function ratio for lithium ions in aqueous solution[J]. The Journal of Physical Chemistry A, 105(3): 602-613. doi: 10.1021/jp001303i [136] YU J J, ZHENG M P, WU Q, et al. , 2015. Natural evaporation and crystallization of Dujiali salt lake water in Tibet[J]. Chemical Industry and Engineering Progress, 34(12): 4172-4178 (in Chinese with English abstract). [137] YU S S, TANG Y, 1981. The hydrochemical characteristics of the saline lakes on the Qinghai-Xizang Plateau[J]. Oceanologia et Limnologia Sinica, 12(6): 498-511 (in Chinese with English abstract). [138] ZHANG L B, CHAN L H, GIESKES J M, 1998. Lithium isotope geochemistry of pore waters from Ocean Drilling Program Sites 918 and 919, Irminger Basin[J]. Geochimica et Cosmochimica Acta, 62(14): 2437-2450. doi: 10.1016/S0016-7037(98)00178-1 [139] ZHANG P X, 1987. Salt lakes in Qaidam Basin[M]. Beijing: Science Press: 1-235 (in Chinese). [140] ZHANG P X, ZHANG B Z, TANG Y, et al. , 1999. Natural resources of salt lakes in China and their development and utilization[M]. Beijing: Science Press: 1-325 (in Chinese). [141] ZHANG W J, TAN H B, XU W S, et al. , 2023. Boron source and evolution of the Zabuye salt lake, Tibet: Indication from boron geochemistry and isotope[J]. Applied Geochemistry, 148: 105516. doi: 10.1016/j.apgeochem.2022.105516 [142] ZHANG Y, TAN H B, CONG P X, et al. , 2022. Boron and lithium isotopic constraints on their origin, evolution, and enrichment processes in a river–groundwater–salt lake system in the Qaidam Basin, northeastern Tibetan Plateau[J]. Ore Geology Reviews, 149: 105110. doi: 10.1016/j.oregeorev.2022.105110 [143] ZHAO Y, MA W P, YANG Y, et al. , 2022. Experimental study on the adsorption of Li+ by clay minerals —implications for the mineralization of clay-type lithium deposit[J]. Acta Mineralogica Sinica, 42(2): 141-153 (in Chinese with English abstract). [144] ZHENG M P, LIU W G, XIANG J, et al. , 1983. On saline lakes in Tibet, China[J]. Acta Geologica Sinica, 57(2): 184-194 (in Chinese with English abstract). [145] ZHENG M P, XIANG J, WEI X J, 1989. Saline lakes on the Qinghai-Xizang (Tibet) Plateau[M]. Beijing: Beijing Science and Technology Publishing Co. , Ltd. : 1-431 (in Chinese). [146] Beijing Mianping Salt Lake Research Ltd., 2006. Exploration report on lithium resource of Damxung Co surface brine in Nima County, Tibet Autonomous Region[R]. (in chinese) [147] ZHENG X Y, YANG S X, 1981. A preliminary study on the constituents of salt lakes in Tibet[J]. Salt Lake Scientific and Technological Information(S1): 8-19 (in Chinese). [148] ZHENG X Y, 1982. The distribution characteristics of B and Li in the brine of Zhacang Caka (Zhangzang Caka) saline lake, Xizang autonomous region, China[J]. Oceanologia et Limnologia Sinica, 13(1): 26-34 (in Chinese with English abstract). [149] ZHENG X Y, YANG S X, 1983. On the components of the saline lake water in Xizang[J]. Oceanologia et Limnologia Sinica, 14(4): 342-352 (in Chinese with English abstract). [150] ZHENG X Y, TANG Y, XU C, 1988. Salt lakes in Xizang[M]. Beijing: Science Press: 1-190 (in Chinese). [151] ZHENG X Y, ZHANG M G, XU C, et al. , 2002. Records of salt lakes in China[M]. Beijing: Science Press: 1-415 (in Chinese). [152] 卞爽, 于志泉, 龚俊峰, 等, 2021. 青藏高原近南北向裂谷的时空分布特征及动力学机制[J]. 地质力学学报, 27(2): 178-194. doi: 10.12090/j.issn.1006-6616.2021.27.02.018 [153] 陈克造, 杨绍修, 郑喜玉, 1981. 青藏高原的盐湖[J]. 地理学报, 36(1): 13-21. doi: 10.3321/j.issn:0375-5444.1981.01.002 [154] 高春亮, 余俊清, 展大鹏, 等, 2009. 柴达木盆地盐湖硼矿资源的形成和分布特征[J]. 盐湖研究, 17(4): 6-13. [155] 李家棪, 1994. 大柴旦盐湖硼、锂分布规律(续)[J]. 盐湖研究, 2(2): 20-28. [156] 刘卫国, 肖应凯, 彭子成, 1999. 柴达木盆地盐湖卤水硼、氯同位素的水化学特性探讨[J]. 盐湖研究, 7(3): 8-14. doi: 10.3969/j.issn.1008-858X.1999.03.002 [157] 刘喜方, 郑绵平, 齐文, 2007. 西藏扎布耶盐湖超大型B、Li矿床成矿物质来源研究[J]. 地质学报, 81(12): 1709-1715. doi: 10.3321/j.issn:0001-5717.2007.12.011 [158] 吕苑苑, 2008. 利用MC-ICP-MS测定硼同位素及其在西藏地热和盐湖中的初步应用[D]. 北京: 中国科学院研究生院: 1-113. [159] 马茹莹, 韩凤清, 马海州, 等, 2015. 青海可可西里盐湖水化学及硼同位素地球化学特征[J]. 地球学报, 36(1): 60-66. doi: 10.3975/cagsb.2015.01.07 [160] 中国地质科学院矿产资源研究所, 2024. 中国钾盐和卤水型锂矿成矿规律研究成果报告[R]. [161] 祁海平, 王蕴慧, 肖应凯, 等, 1993. 中国盐湖中硼同位素的初步研究[J]. 科学通报, 38(7): 634-637. [162] 卿德林, 马海州, 李斌凯, 2012. 班戈错Ⅱ湖晶间卤水蒸发硼浓度及硼同位素分馏研究[J]. 盐湖研究, 20(3): 15-20. [163] 宋鹤彬, 李亚文, 1994. 中国南海海水蒸发实验过程中地球化学行径[J]. 地球学报, 15(1-2): 157-167. [164] 孙大鹏, 1991. 柴达木盆地小柴旦湖硼酸盐的形成[J]. 矿物岩石, 11(4): 57-65. [165] 孙大鹏, 唐渊, 许志强, 等, 1991. 青海湖湖水化学演化的初步研究[J]. 科学通报, 36(15): 1172-1174. [166] 孙大鹏, 肖应凯, 王蕴慧, 等, 1993. 青海湖硼同位素地球化学初步研究[J]. 科学通报, 38(9): 822-825. [167] 佟伟, 章铭陶, 张知非, 等, 1981. 西藏地热[M]. 北京: 科学出版社. [168] 万红琼, 孙贺, 刘海洋, 等, 2015. 俯冲带Li同位素地球化学: 回顾与展望[J]. 地学前缘, 22(5): 29-43. [169] 王庆忠, 肖应凯, 张崇耿, 等, 2001. 青海和西藏的某些天然硼酸盐矿物的硼同位素组成[J]. 矿物岩石地球化学通报, 20(4): 364-366. doi: 10.3969/j.issn.1007-2802.2001.04.044 [170] 王苏民, 窦鸿身, 1998. 中国湖泊志[M]. 北京: 科学出版社: 1-580. [171] 吴俐俐, 马文展, 唐渊, 1984. 青藏高原高硼卤水的水化学特征及其成因[J]. 地理研究, 3(4): 1-11. [172] 伍倩, 郑绵平, 乜贞, 等, 2012. 西藏当雄错碳酸盐型盐湖卤水自然蒸发析盐规律研究[J]. 无机化学学报, 28(9): 1895-1903. [173] 伍倩, 郑绵平, 乜贞, 等, 2013. 西藏当雄错盐湖卤水冬季日晒蒸发实验研究[J]. 地质学报, 87(3): 433-440. [174] 吴雅琴, 赵志琦, 2011. 高岭石和蒙脱石吸附Li+的实验研究[J]. 矿物学报, 31(2): 291-295. [175] 吴中海, 张永双, 胡道功, 等, 2007. 西藏错那—沃卡裂谷带中段邛多江地堑晚新生代正断层作用[J]. 地质力学学报, 13(4): 297-306. [176] 肖应凯, 祁海平, 王蕴慧, 等, 1994. 青海大柴达木湖卤水、沉积物和水源水中的锂同位素组成[J]. 地球化学, 23(4): 329-338. [177] 肖应凯, SHIRODKAR P V, 刘卫国, 等, 1999. 青海柴达木盆地盐湖硼同位素地球化学研究[J]. 自然科学进展, 9(7): 612-618. doi: 10.3321/j.issn:1002-008X.1999.07.007 [178] 余疆江, 郑绵平, 伍倩, 等, 2015. 西藏杜佳里盐湖湖水的自然蒸发及析盐规律[J]. 化工进展, 34(12): 4172-4178. [179] 于昇松, 唐渊, 1981. 青藏高原盐湖的水化学特征[J]. 海洋与湖沼, 12(6): 498-511. [180] 张彭熹, 1987. 柴达木盆地盐湖[M]. 北京: 科学出版社: 1-235. [181] 张彭熹, 张保珍, 唐渊, 等, 1999. 中国盐湖自然资源极其开发利用[M]. 北京: 科学出版社: 1-325. [182] 赵越, 马万平, 杨洋, 等, 2022. 黏土矿物对Li+的吸附实验研究: 对黏土型锂矿成矿启示[J]. 矿物学报, 42(2): 141-153. [183] 郑绵平, 刘文高, 向军, 等, 1983. 论西藏的盐湖[J]. 地质学报, 57(2): 184-194. [184] 郑绵平, 向军, 魏新俊, 1989. 青藏高原盐湖[M]. 北京: 北京科学技术出版社: 1-431. [185] 北京绵平盐湖研究院, 2006. 西藏自治区尼玛县当雄错表面卤水锂矿勘查报告[R]. [186] 郑喜玉, 杨绍修, 1981. 西藏盐湖物质成分的初步研究[J]. 盐湖科技资料(S1): 8-19. [187] 郑喜玉, 1982. 西藏扎仓茶卡盐湖卤水硼、锂的分布特征[J]. 海洋与湖沼, 13(1): 26-34. [188] 郑喜玉, 杨绍修, 1983. 西藏盐湖成分及其成因探讨[J]. 海洋与湖沼, 14(4): 342-352. [189] 郑喜玉, 唐渊, 徐昶, 1988. 西藏盐湖[M]. 北京: 科学出版社: 1-190. [190] 郑喜玉, 张明刚, 徐昶, 等, 2002. 中国盐湖志[M]. 北京: 科学出版社: 1-415. -



 下载:
下载: