EVALUATION OF THE METHODS FOR QUANTITATIVE DETERMINATION OF FE2+/FE3+ RATIOS OF MAGNETITE FROM QIMANTAG METALLOGENIC BELTS
-
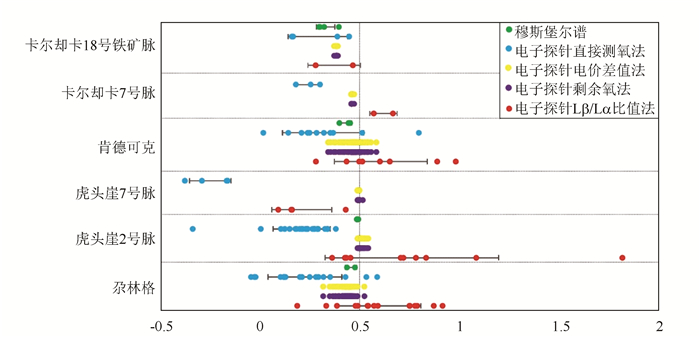
摘要: 为了厘清磁铁矿成分测试过程中Fe2+/Fe3+比值分析各种方法的准确性及适用范围;采用直接测氧法、Lβ/Lα强度比值法、电价差值法、剩余氧法和穆斯堡尔谱法,对祁漫塔格成矿带中典型矿床中磁铁矿的Fe2+/Fe3+比值进行了研究,结果表明电价差值法、剩余氧法和穆斯堡尔谱法是相对比较准确的测试方法,但穆斯堡尔谱法不是原位分析方法,存在适用范围的缺陷。Abstract: Through the determination of Fe2+/Fe3+ ratios of magnetite from the Qimantag metallogenic belt, the quality of five different methods is evaluated. Oxygen as a super light element, affects the determination results of FeO and Fe2O3 observably when direct oxygen measurement method is used. The valence state method, based on Lα and Lβ spectral intensity ratio of Fe, is also unreliable for FeO and Fe2O3 measurements, because it is hard to establish the relationship between Lβ/ Lα (spectral intensity ratio) and Fe2+/Fe3+(content ratio). Relatively, the charge difference method, the surplus-oxygen method and Mssbauer spectroscopy are still the most favorable methods; however, Mssbauer spectroscopy is limited in spatial resolution to ca. 200 mm, obliterating potential zonations in Fe2+/ Fe3+ ratio.
-
[1] 陈光远, 邵伟, 孙岱生.胶东金矿成因矿物学与找矿[M].重庆:重庆出版社, 1989.CHEN Guangyuan, SHAO Wei, SUN Daisheng. Genesis mineralogy and prospecting mineralogy in Jiaodong gold mine[M]. Chongqing: Chongqing Press, 1989. (in Chinese) [2] 陈光远, 孙岱生, 殷辉安.成因矿物学与找矿矿物学[M].重庆:重庆出版社, 1987: 1-874CHEN Guangyuan, SUN Daisheng, YIN Huian. Genesis mineralogy and prospecting mineralogy[M]. Chongqing: Chongqing Press, 1987: 1-874. (in Chinese) [3] 周剑雄, 毛水和, 陈克樵.电子探针分析[M].北京:地质出版社, 1988.ZHOU Jianxiong, MAO Shuihe, CHEN Keqiao. Electron probe analysis[M]. Beijing: Beijing Press, 1988. (in Chinese) [4] 陈克樵, 欧阳菲.电子探针定量分析直接测定含铁矿物中二价和三价铁[J].岩矿测试, 1992, 11(4): 306-310. http://www.cnki.com.cn/Article/CJFDTOTAL-YKCS199204002.htmCHEN Keiqiao, OUYANG Fei. Determination of Iron(Ⅱ) and Iron (Ⅲ) in iron-bearing minerals by electron probe analysis[J]. Rock and Mineral Analysis, 1992, 11(4): 306-310 (in Chinese with English abstract). http://www.cnki.com.cn/Article/CJFDTOTAL-YKCS199204002.htm [5] 合志阳一.电子线分光法的状态分析[M]. 1981.HE Z Y Y. State analysis of electron beam spectrometry[M]. 1981 (in Japanese). [6] PERRY E C, TAN F C, MOREY G B. Geology and stable isotope geochemistry of the biwabik iron formation, northern Minnesota[J]. Economic Geology, 1973, 68(7): 110-1125. DOI: 10.2113/gsecongeo.68.7.1110. [7] HEMINGWAY B S. Thermodynamic properties for bunsenite, NiO, magnetite, Fe3O4, and hematite, Fe2O3, with comments on selected oxygen buffer reactions[J]. American Mineralogist, 1990, 75(7): 781-790. [8] BARLEY M E, PICKARD A L. An extensive, crustally-derived, 3325 to 3310 Ma silicic volcanoplutonic suite in the eastern Pilbara Craton: evidence from the Kelly Belt, McPhee dome and Corunna downs batholith[J]. Precambrian Research, 1999, 96(1-2): 41-62. DOI: 10.1016/S0301-9268(99)00003-0. [9] MARSCHIK R. The Candelaria-Punta del cobre iron oxide Ci-Au(-Zn-Ag) Deposits, Chile[J]. Economic Geology, 2001, 96(8): 1799-1826. DOI: 10.2113/96.8.1799. [10] CORNELL S E, JICKELLS T D, CAPE J N, et al. Organic nitrogen deposition on land and coastal environments: a review of methods and data[J]. Atmospheric Environment, 2003, 37(16): 2173-2191. DOI: 10.1016/S1352-2310(03)00133-X. [11] DE HALLER A, FONTBOTE L. The raul-condestable iron oxide copper-gold deposit, central coast of Peru: ore and related hydrothermal alteration, sulfur isotopes, and thermodynamic constraints[J]. Economic Geology, 2009, 104(3): 365-384. DOI: 10.2113/gsecongeo.104.3.365. [12] OHMOTO H. Nonredox transformations of magnetite-hematite in hydrothermal systems[J]. Economic Geology, 2003, 98(1): 157-161. DOI: 10.2113/gsecongeo.98.1.157. [13] OTAKE H, EGAMI S, OHTA H, et al. GaN-based trench gate metal oxide semiconductoe field effect transistor with over 100 cm2/(V s) channel mobility[J]. Japanese Journal of Applied Physics, 2007, 46(25): L599-L601. DOI: 10.1143/JJAP.46.1599. -




 下载:
下载: